You can't make a claim about a drug being "safe" without the science to back it up—if you're a drug maker or an "investigator" of the drug. The FDA doesn't allow drug makers or investigators to make that claims like that without proof. But if you're not a drug maker or an "investigator," the FDA won't stop you from making claims about a drug's safety. Which is why this is being allowed to happen:
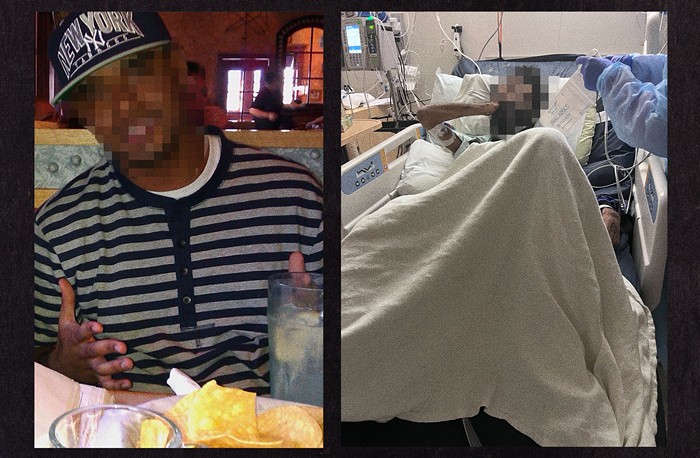
Dex is being offered off-label by some clinicians to pregnant women who, through genetic counseling, are identified as being "at risk" for giving birth to a child with a disease called Congenital Adrenal Hyperplasia (CAH). CAH is a lifelong disease that requires endocrine management. The child exposed to prenatal dex will still be born with the disease of CAH, because the prenatal treatment does nothing to prevent the disease. So what is the prenatal dex aimed at? In some forms of CAH, girls are born with genitals that are between the male and female types. If a woman is pregnant with a girl fetus affected by this type of CAH and takes dex starting very early in the pregnancy, the girl may be born with genitals closer to the typical female's.
Particularly since this off-label prenatal use does nothing to prevent CAH, the medical establishment has been understandably worried about this. When you start an off-label drug use as early as the 5th-8th week of pregnancy, and you intend that drug to cross the placental barrier and you intend to change the fetus's development, a lot of unintended harm can occur....
[The] major proponent of this use, Dr. Maria New of Mount Sinai Medical Center, tells patients on her Foundation website that "with nearly 20 years' experience, the treatment has been found safe for mother and child." But has it? Here's what the latest major medical consensus group in fact found: "The task force was hampered by the lack of high-quality data. Of 1,083 studies originally identified [by the task force] only four met the [scientific] quality criteria agreed upon by the sponsoring groups. [...] Outcome data on prenatal treatment are suspect. Most are derived from questionnaires, not from physical examination of the offspring [i.e., the children treated in utero]. And because prenatal dexamethasone treatment is relatively new, no offspring have yet reached middle age where many problems can be expected to present."
About ten years ago, a committee of the American Academy of Pediatrics (AAP) was so disturbed by Dr. New's approach to prenatal dex that they warned her in the journal Pediatrics: "The maxim of 'first do no harm' requires a cautious, long-term approach, which is why the Academy Committee unanimously agrees that prenatal glucocorticoid therapy for CAH should be confined to centers doing controlled prospective, long-term studies. The memory of the tragedies associated with prenatal use of DES and thalidomide demands no less." In other words, the AAP was worried that prenatal dex might, in the long run, turn out like DES or thalidomide.